Laboratory
Robust and precise characterisation of cellular phenotypes
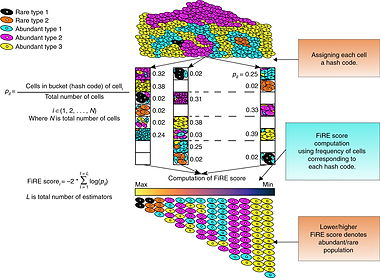
Our recently developed ability to peep into molecular portraits of individual cells provides an exceptional resolution for viewing the emergence, dynamics, and fate of cellular phenotypes. However to extract all this, one has to tackle the large dimensions and noisy readings associated with the single-cell omics readouts. Our lab specializes in developing computational methods to tackle these problems. In the past, we developed some of the speediest, resource-friendly, and accurate computational methods for single-cell clustering (dropClust, NAR 2018a), rare cell detection (FiRE, Nat. Comm. 2018), cell atlas searching (CellAtlasSearch, NAR 2018b), differential expression analysis (ROSeq, Genome Res. 2021), etc. These methods fuse elements of statistics, machine learning, and Big Data algorithms. Currently, we are leveraging our experience in handling single-cell transcriptomics data to solve higher-order mysteries, such as non-genetic signatures of competitiveness among cancer clones, and linking RNA stability with cell-signaling dynamics (bioRxiv 2021).

Cancer detection and treatment

We developed a blood-based cancer diagnostic test using Tumor Educated Platelets (TEPs), which accurately identifies cancer with minimal infrastructure requirements. This test is cost-effective, works across multiple cancer types, and delivers rapid results, making it accessible even in basic diagnostic labs.
On the therapeutic side, we’ve created AI models that predict patient-specific responses to cancer drugs. By integrating gene expression data and drug descriptors, our models offer reliable predictions, even for drugs not included in the training datasets, paving the way for more precise and adaptive cancer therapies.
Company website
Hunting expression based hints to cancer from blood and tracking cancer-immune interaction dynamics
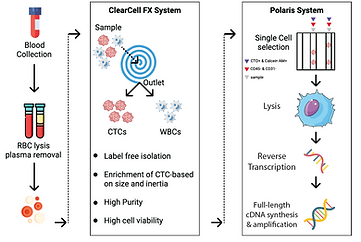
Our obsession with gene expression, landed us into cancer since cancers are gene expression kaleidoscopes and a beautiful system to explore RNA elasticity. In the context of cancer, there are a few unsolved paradigms that interest us the most. One of those is how to recognize all phenotypes of Circulating Tumour Cells that float around in peripheral blood (JCM 2020) (bioRxiv 2021). As we know cancer cells undergo molecular remodeling to successfully traverse distant organs. Only a fraction of such disguising strategies is known to us. One is Epithelial to Mesenchymal Transition or EMT. Current best practice methods are limited to selection for size or canonical markers such as EpCAM. We use a unique combination of unbiased, microfluidic enrichment of CTCs and gene expression pattern recognition to detect CTCs irrespective of any specific marker. Along similar lines, we developed and commercialized the Tumour Educated Platelet (TEP) gene panel for early cancer detection (BMC Genomics 2021) (CareOnco). More recently we interrogated NK-Cancer cell interaction at doublet resolution and learned signatures associated with killing by NKs. Notably, not every NK cell kills every cancer cell that comes in its contact. A different ongoing study alludes to single cell-based signatures that are associated with clonal advantage within a tumor ecosystem. Watch out for future developments.